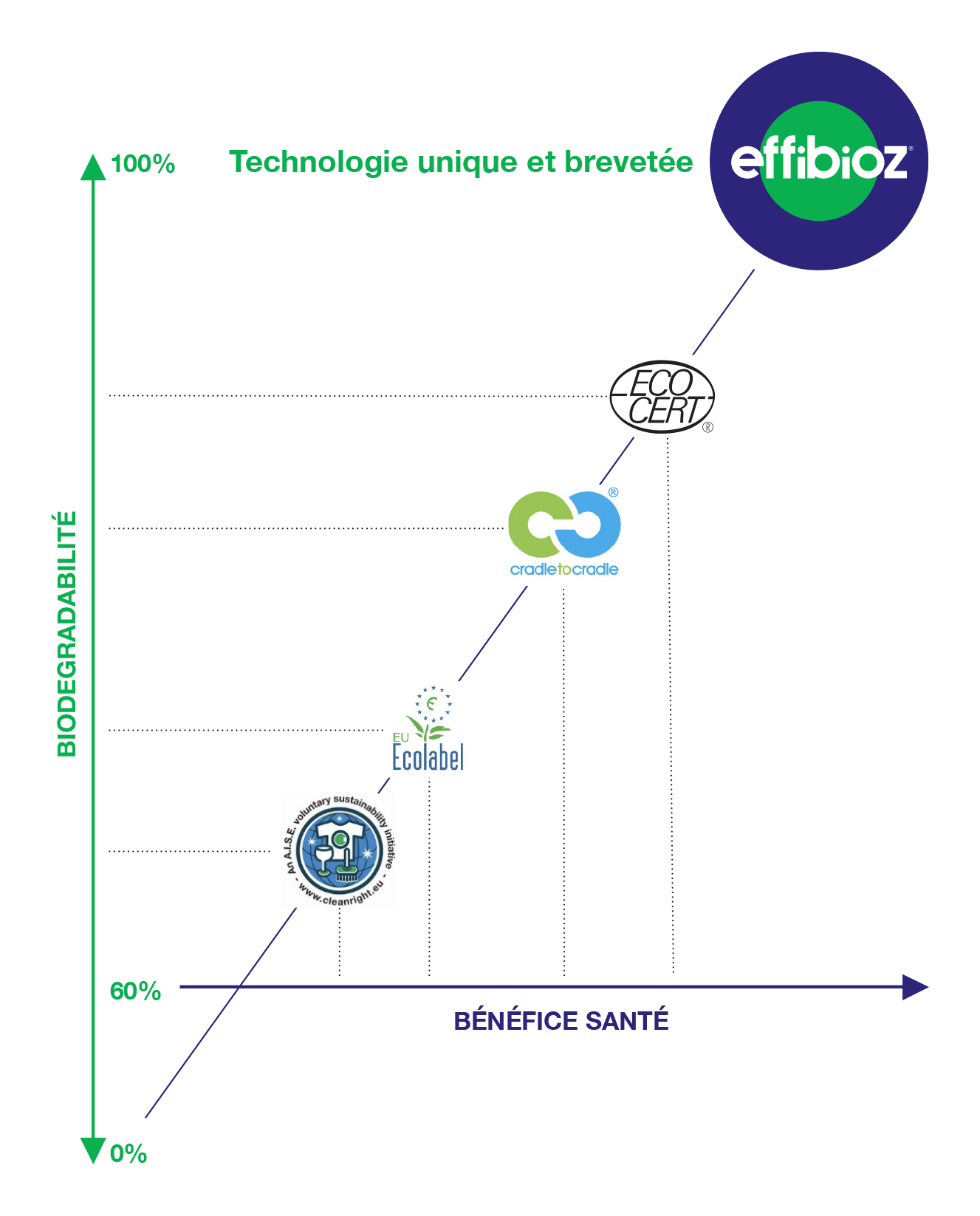
The Effibioz patent,
when necessity
is the law.
is 0% of chemical
chemical-synthetic residue
Effibioz disinfectants are subject to much more stringent legislation than conventional disinfectants and other hydro-alcoholic gels. They guarantee above all an optimum efficiency evaluated by independent authorities such as the European authorities like the ECHA (European Chemicals Agency) which ensures the conformity of our products according to the new European biocide regulation (BPR), and in particular the ANSES for France, as well as the DGS (Direction Générale de la Santé) and the DGPR (Direction Générale de la Prévention des Risques)
The effibioz patent also avoids harmful effects on the skin: dryness, redness, burns, allergies, sensitization, chemical permeability, destruction of the natural immune barrier. “With the daily use of hydroalcoholic gels, we have hands on fire,” said a nurse on Twitter in April 2020.
It was therefore urgent to find an effective solution to the harmful effects of alcohol on the hands.
Consumer protection begins with information and knowledge, and therefore with clear labelling.

BPR – Article 1: Preservation of human and animal health and the environment and harmonization of rules concerning the placing on the market and use of biocidal products
BPR – Article 25: …very demanding safety and efficacy conditions for products to be eligible for an AMMS procedure.
BPR – Articles 69 & 72: does not include the words “low risk product”, “non-toxic”, “does not harm health”, “natural”, “environmentally friendly”, “animal friendly”.
EGalim – Article 76: products under AMMS are exempted from the prohibitions, and therefore correspond to products with the lowest risk.
Effibioz disinfectants are subject to a much more stringent legislation than conventional disinfectants and other hydro-alcoholic gels. They guarantee above all an optimum efficiency evaluated by independent authorities such as the European authorities like the ECHA (European Chemicals Agency) which ensures the compliance of our products according to the new European Biocide Regulation (BPR), and in particular the ANSES for France, as well as the DGS (General Directorate of Health) and the DGPR (General Directorate of Risk Prevention).
The Effibioz patent also avoids harmful effects on the skin: dryness, redness, burns, allergies, sensitization, chemical permeability, destruction of the natural immune barrier, “With the daily use of hydroalcoholic gels, our hands are on fire” testifies a nurse on Twitter, in April 2020.
It was therefore urgent to find an effective solution to the harmful effects of alcohol on the hands.